Lee S. Sunderlin
Representative Publications
The bond dissociation energies of SO3–X− (X = F, Cl, Br and I). Hao, C.; Gilbert, T. M.; Sunderlin, L. S.(2005) Can. J. Chem., 83: 2013–2019.
Measuring basicities of amino acids using an ion‐trap mass spectrometer: A physical chemistry laboratory experiment. Sunderlin, L. S.; Ryzhov, V.; Keller, L. M. M.; Gailard, E. R. (2005) J. Chem. Educ., 82: 1071–1073.
The effect of substituents on the strength of A–Cl− (A = Si, Ge, and Sn) bonds in hypervalent systems: ACl5−, ACl4F−, and A(CH3)3Cl2−. Hao, C.; Kaspar, J. D.; Check, C. E.; Lobring, K. C.; Gilbert, T. M.; Sunderlin, L. S. (2005) J. Phys. Chem. A, 109: 2026–2034.
The dissociation energy of Cl2O2+. Bailey, J. M.; Hao, C.; Johnson, B. J.; Sunderlin, L. S. (2005) Int. J. Mass Spectrom., 241: 143–148.
Bond strengths in POCl3−, POCl2−, and PSCl2−. Lobring, K. C.; Check, C. E.; Boggs, M. L.; Keating, P. R.; Sunderlin, L. S. (2005) Int. J. Mass Spectrom., 241: 75–81.
The isozahlic and additivity rules: Estimation of ion volumes—A route to the energetics and entropics of new and traditional ionic materials. Jenkins, H. D. B.; Glasser, L.; Klapötke, T. M.; Crawford, M. J.; Lee, J.; Schrobilgen, G. J.; Sunderlin, L. S.; Liebman, J. S. (2004) Inorg. Chem., 43: 6238–6248.
Effect of substituents on the strength of hypervalent phosphorus–halogen bonds. Check, C. E.; Lobring, K. C.; Keating, P. R.; Gilbert, T. M.; Sunderlin, L. S.(2003) J. Phys. Chem. A, 107: 8961–8967.
New measurements of the thermochemistry of SF5−and SF6−. Lobring, K. C.; Check, C. E.; Gilbert, T. M.; Sunderlin, L. S. (2003) Int. J. Mass Spectrom., 222: 221–227.
Gas‐Phase Kinetics and Thermochemistry
The flowing afterglow (FA) was developed in the 1960s to study the kinetics of ion–molecule reactions in the atmosphere. Since then, other researchers have developed more elaborate versions of the basic apparatus and studied an increasingly broad array of chemical problems.
The instrument we have constructed at NIU is a unique multiple‐temperature flowing afterglow‐tandem mass spectrometer. The tandem mass spectrometer is used primarily for collision‐induced dissociation (CID), where bond strengths are determined by measuring the translational energy necessary to drive an endothermic reaction. For these experiments, an FA is an ideal ion source, since it can be used to create ions with well‐defined distributions of internal energy.
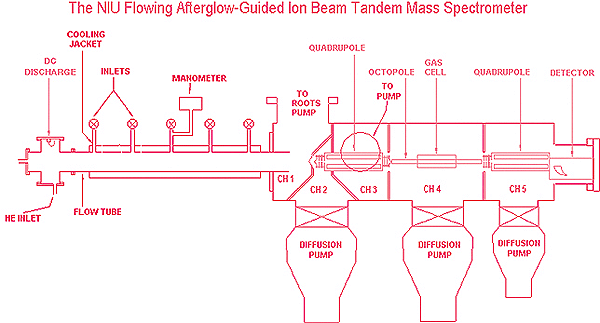 Schematic diagram of the flowing afterglow‐tandem mass spectrometer.
Schematic diagram of the flowing afterglow‐tandem mass spectrometer.
One major field of study is hypervalent ions, such as I3−, where the central atom has more than eight electrons in its valence shell. The electronic structure of these species is still controversial. (For example, are d orbitals involved or not?) The relevant ions can be synthesized in the FA; CID gives the bond strengths in these ions. Hypervalent ions are excellent test models for the study of solvation and computational techniques; these have practical significance in solution, solid‐state and even atmospheric chemistry.
Another field of interest is the chemistry of oxyacids. The conversion of elements such as sulfur into oxidized forms such as sulfuric acid has a substantial impact on the Earth's atmosphere. Surprisingly little is known about these processes. Using the instrument described previously, it is possible to measure thermochemistry, reaction barriers and the effects of catalysts on systems of direct interest and on model systems. These studies also involve close interactions with computational chemists.

Associate Professor
Director of Undergraduate Studies
La Tourette Hall 327
815‐753‐6870
sunder@niu.edu
Educational Background
Research Associate, Purdue University, 1990–1994
Ph.D., University of California, Berkeley, 1990
NSF Predoctoral Fellow, 1985–1988
B.S., California Institute of Technology, 1984
Research Interests
Mass spectrometry; flowing afterglow studies of gas‐phase kinetics and thermochemistry; hypervalent bonding; atmospheric chemistry.