Evgueni E. Nesterov
Representative Publications
- Barashkin, A. A.; Amadou, H.; Nesterov, E. E. "Photochemistry with plane-polarized light: Controlling selectivity of a photochemical rearrangement." Chemistry – A European Journal, 2024, 30, e20240338.
- Howell, M. T.; Fronczek, F. R.; Nesterov, E. E. "MIDA boronate monomers in Suzuki-Miyaura catalyst-transfer polymerization: The pros and cons." Macromolecules, 2024, 57, 7847–7861.
- Howell, M. T.; Kei, P.; Anokhin, M. V.; Losovyj, Y.; Fronczek, F. R.; Nesterov, E. E. "Suzuki-Miyaura catalyst-transfer polymerization: New mechanistic insights." Polymer Chemistry, 2023, 14, 4319–4337.
- Mohamed, M.; Klenke, A. K.; Anokhin, M. V.; Amadou, H.; Bothwell, P. J.; Conroy, B.; Nesterov, E. E.; Nesterova, I. V. "Zero-background small-molecule sensors for near-IR fluorescent imaging of biomacromolecular targets in cells." ACS Sensors, 2023, 8, 1109–1118.
- Kei, P.; Howell, M. T.; Chavez, C. A.; Mai, J. C.; Do, C.; Hong, K.; Nesterov, E. E. "Kinetically controlled formation of semi-crystalline conjugated polymer nanostructures." Macromolecules, 2021, 54, 2162–2177.
- Ducharme, G. T.; LaCasse, Z.; Sheth, T.; Nesterova, I. V.; Nesterov, E. E. "Design of turn-on near-infrared fluorescent probes for highly sensitive and selective monitoring of biopolymers." Angewandte Chemie International Edition, 2020, 59, 8440–8444.
- Wang, C.-H.; Nesterov, E. E. "Amplifying fluorescent conjugated polymer sensor for singlet oxygen detection." Chemical Communications, 2019, 55, 8955–8958.
- Chatterjee, S.; Karam, T. E.; Rosu, C.; Wang, C.-H.; Youm, S. G.; Li, X.; Do, C.; Losovyj, Y.; Russo, P. S.; Haber, L. H.; Nesterov, E. E. "Silica–conjugated polymer hybrid fluorescent nanoparticles: Preparation by surface-initiated polymerization and spectroscopic studies." The Journal of Physical Chemistry C, 2018, 122, 6963–6975.
- Chiang, C.-H.; Pangeni, D.; Nesterov, E. E. "Higher energy gap control of fluorescence in conjugated polymers: Turn-on amplifying chemosensor for hydrogen sulfide." Macromolecules, 2017, 50, 6961–6966.
- Youm, S. G.; Hwang, E.; Chavez, C. A.; Li, X.; Chatterjee, S.; Lusker, K. L.; Lu, L.; Strzalka, J.; Ankner, J. F.; Losovyj, Y.; Garno, J. C.; Nesterov, E. E. "Polythiophene thin films by surface-initiated polymerization: Mechanistic and structural studies." Chemistry of Materials, 2016, 28, 4787–4804.
Functional Organic Materials and Polymers
The development of functional organic materials is a rapidly growing area of science, promising to replace traditionally used materials with cheaper and better-performing alternatives. Often, it brings about new applications that have never been considered before. In our group, we pursue an approach that starts from a thorough design of a molecule possessing a desired property, and then we study the performance of this compound as part of a bulk material or device. Many of our materials are designed to be controlled by light. Such materials may find use in nanoscale electronics, photonics, chemical and biological sensors, biomedical research, and various other fields. This multidisciplinary program combines contemporary and traditional areas of physical organic and synthetic organic chemistry, theoretical and computational chemistry, and materials and macromolecular chemistry. Some of the current projects are outlined below.
Controlled Polymerization for the Preparation of Conjugated Polymers
Conjugated polymers represent a unique class of organic materials foundational in various applications, spanning from chemo- and biosensing devices to light-emitting diodes and lasers with tunable emission colors. We are developing controlled polymerization strategies that can be used for the preparation of structurally precise complex conjugated polymer systems. Catalyst-transfer polymerization (CTP) based on Pd-catalyzed Suzuki-Miyaura cross-coupling reaction is currently one of the most promising methods toward achieving such a goal. Further expansion and development of practical applications of CTP methods will hinge on a clear mechanistic understanding of both the entire process and the particular steps involved in the catalytic cycle. Therefore, mechanistic studies of the Suzuki-Miyaura CTP process are a major part of our current research efforts.
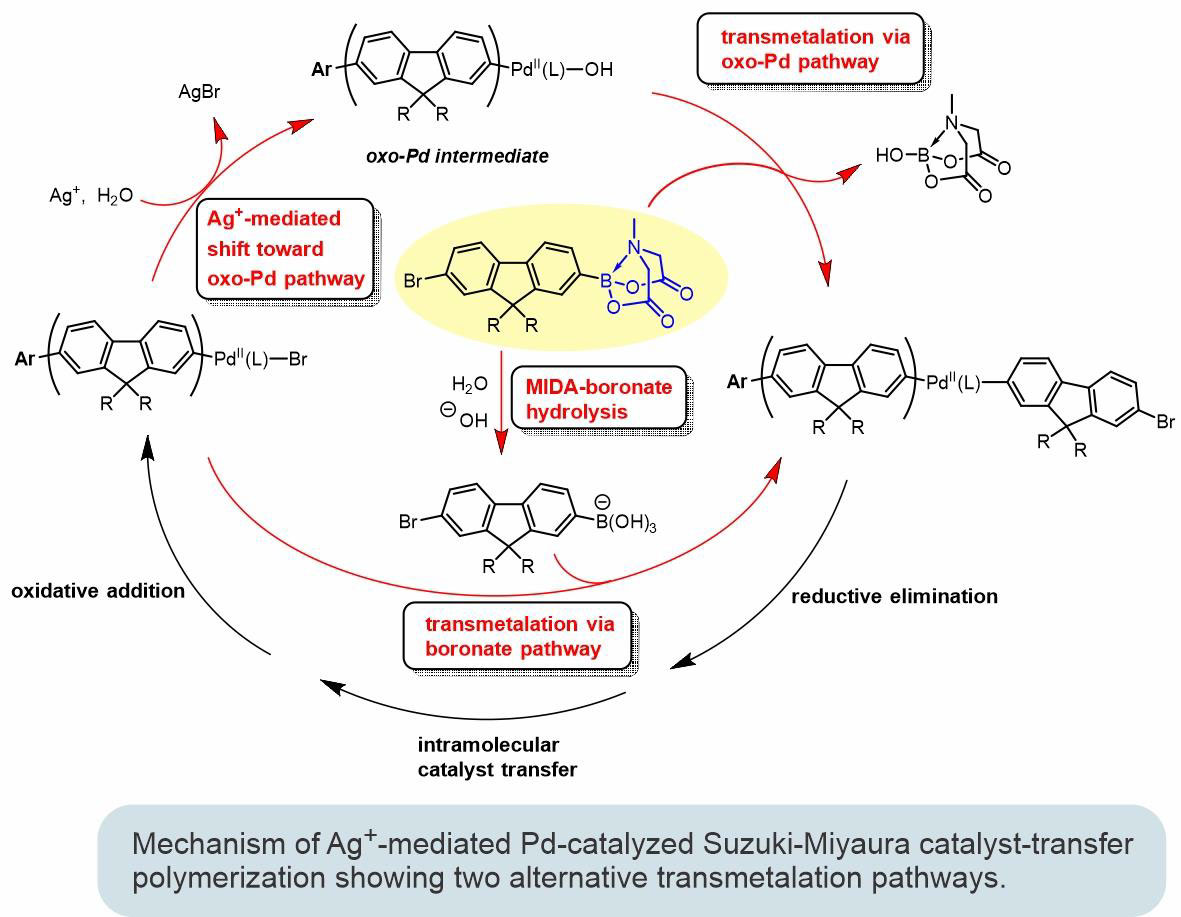
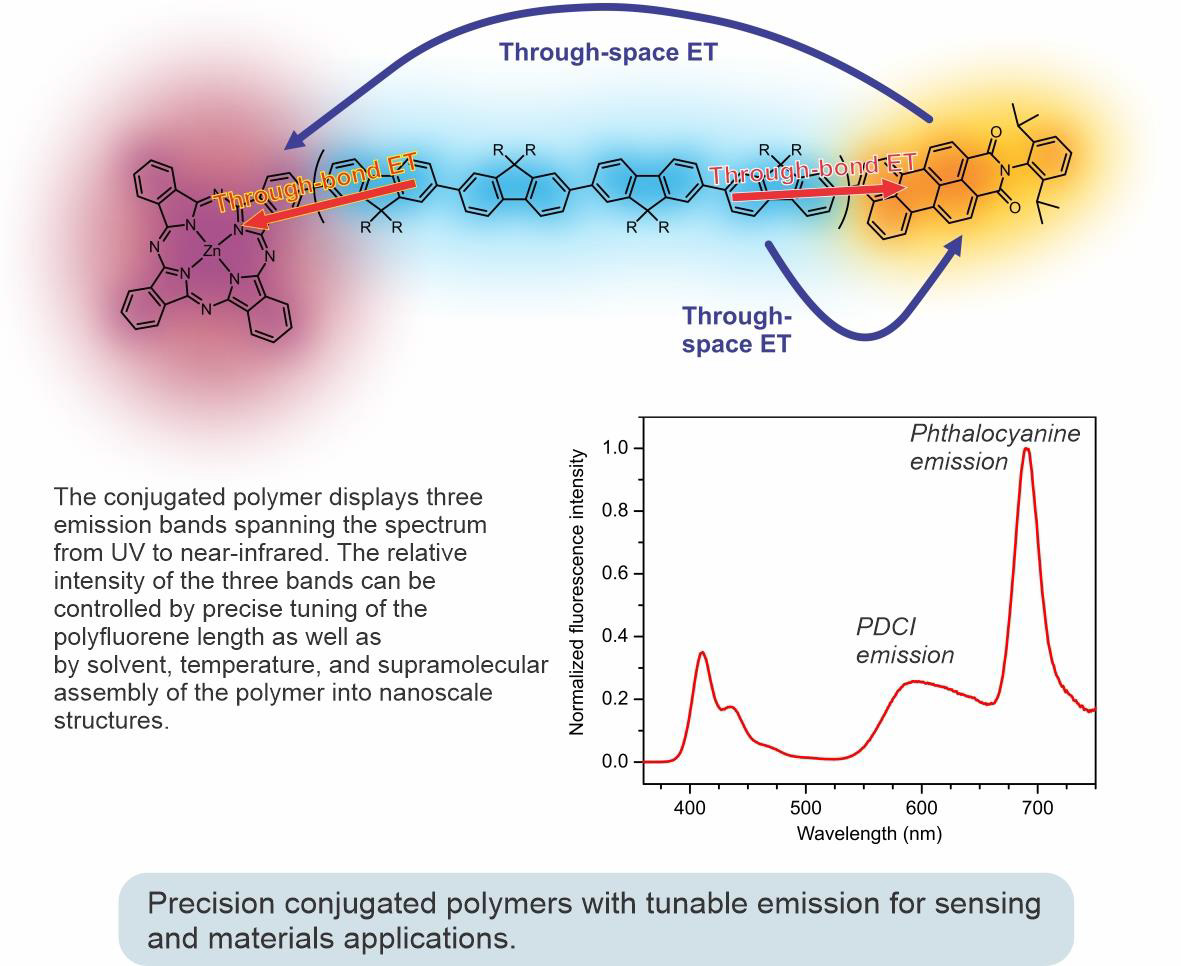
Using controlled polymerization strategies recently developed in our group, we succeeded in preparing a series of well-defined fluorescent conjugated polymers with tunable emission colors, like the one shown in the figure below. This polymer incorporates a phthalocyanine unit at one end and a perylenedicarboximide (PDCI) unit at the other end. A complex interplay between different energy transfer pathways brings about the possibility of controlling the emission color of the polymer by external stimuli (such as temperature or solvent) or through supramolecular assembly to nano- and mesoscale architectures.
Signal-Amplifying Fluorescent Chemosensors
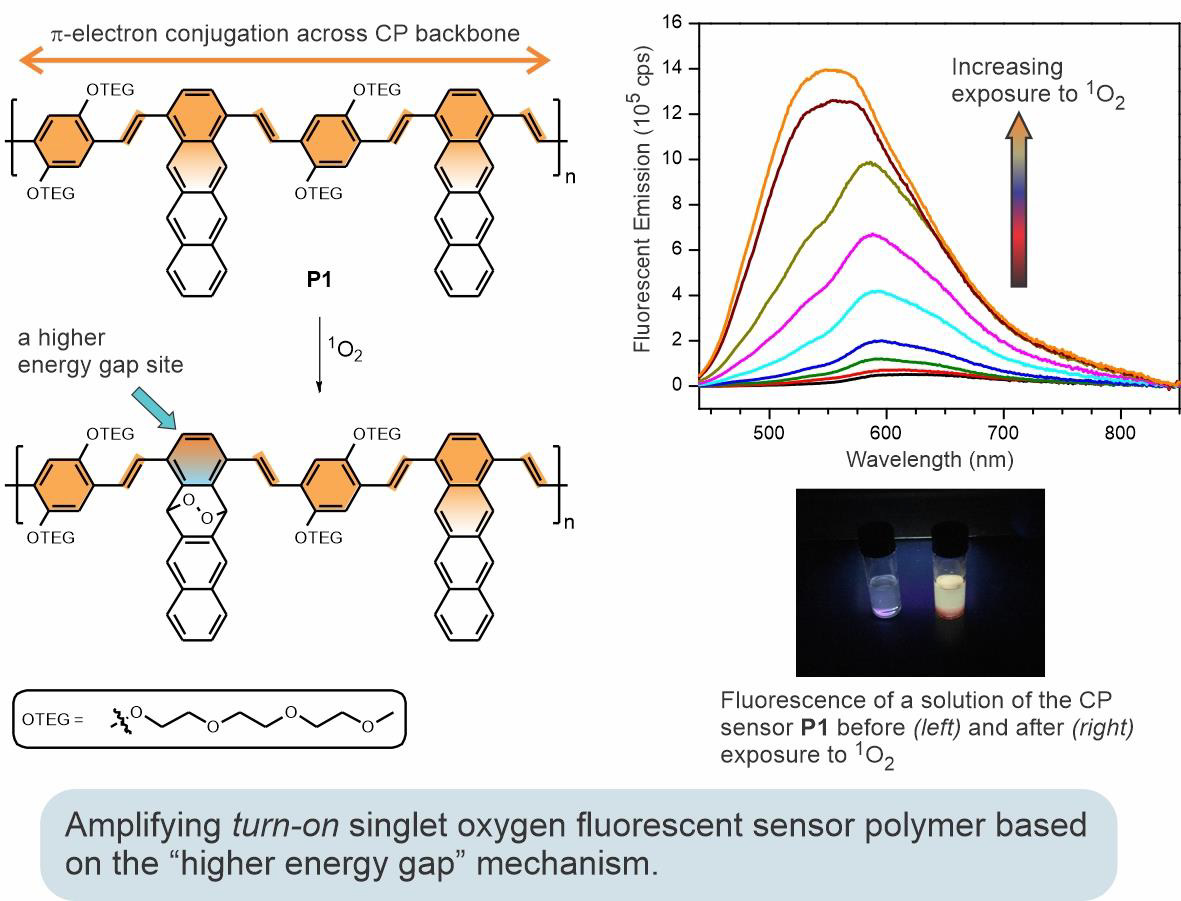
In a quest to develop a universal approach to signal-amplifying fluorescent chemosensors, we are studying the "higher energy gap" mechanism. This mechanism is based on the modulation of intramolecular energy transfer in fluorescent conjugated polymers through the formation of a higher energy gap "roadblock" upon reaction with a target analyte. Shown below is an example of an efficient turn-on amplifying sensor for singlet oxygen—an important biomedical and environmental monitoring analytical target. The polymer sensor incorporates 1,4-disubstituted tetracene units, which act as reactive sites for singlet oxygen. The resulting polymer sensor demonstrates significant fluorescent signal amplification and a broad analyte detection range required for practical applications.
Small-Molecule Biosensors for Near-Infrared Imaging
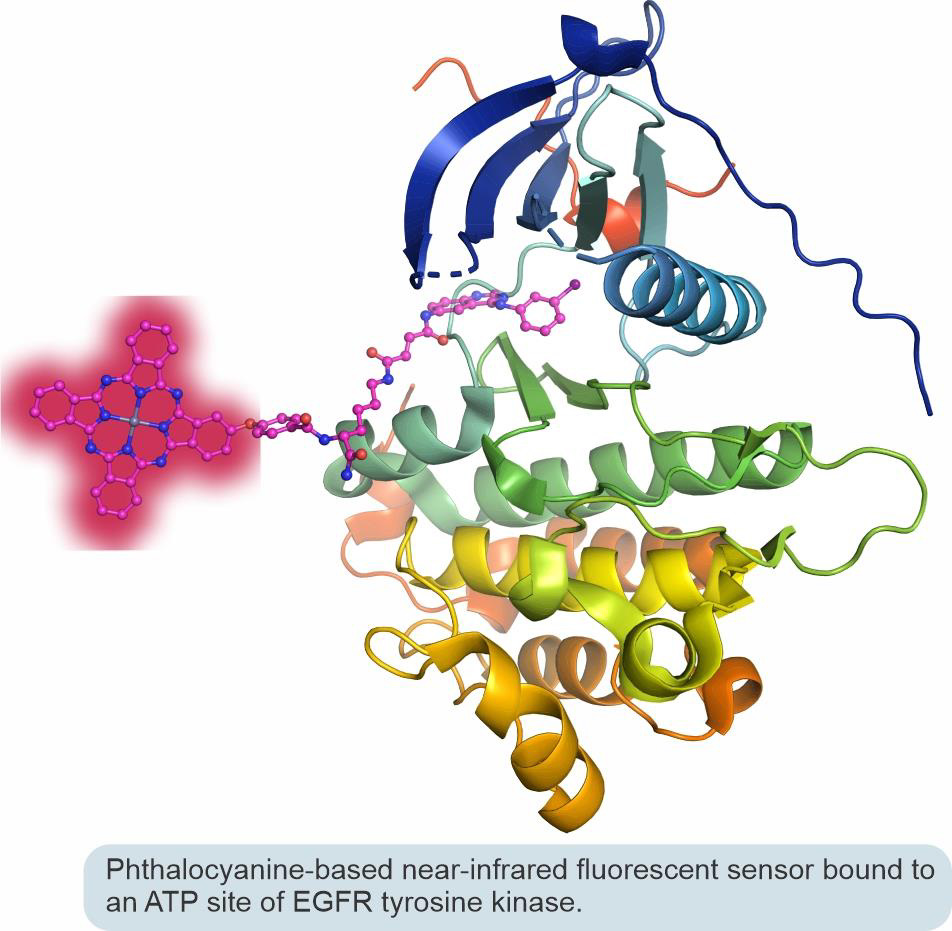
In collaborative efforts with Professor Irina Nesterova from our department, we designed a new generation of small-molecule biosensors that produce zero background but become brightly fluorescent in the near-infrared spectral range upon selective interaction with a biomolecular target. These sensors operate using a fluorescence turn-on mechanism based on the aggregation/deaggregation of phthalocyanine chromophores. Specifically, we research sensors that can be used for in-cell visualization of epidermal growth factor receptor (EGFR) tyrosine kinase and for the rapid and reliable screening of small-molecule EGFR kinase inhibitors used in personalized anticancer medical treatments.
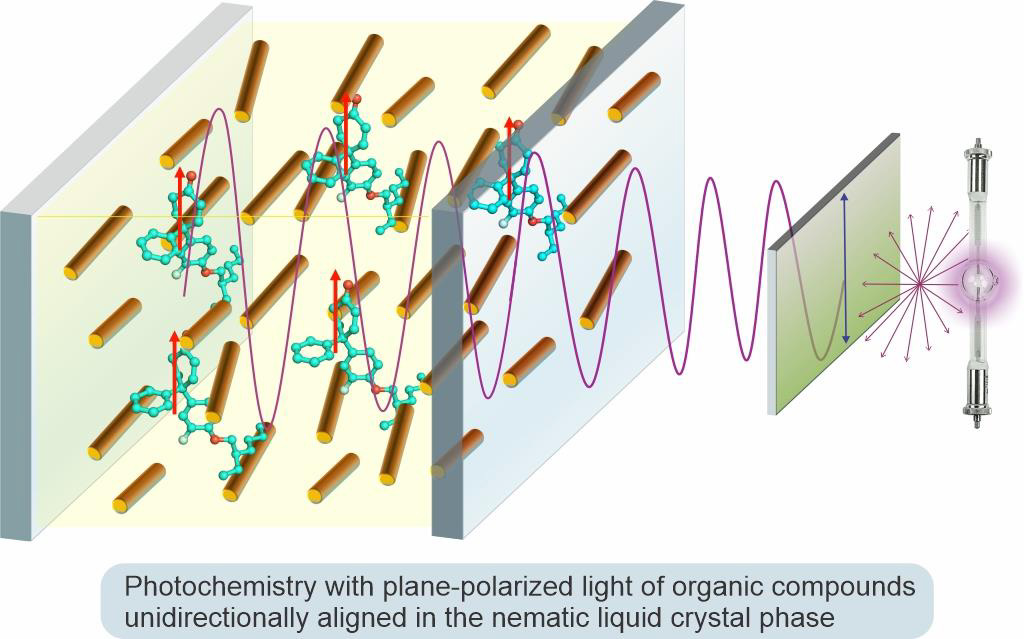
Organic Photochemistry with Plane-Polarized Light
Organic photochemistry offers reactions that can potentially lead to new environmentally benign chemical processes relying on light as a source of energy for chemical transformations. Over the past 50 years, a principal approach to controlling conventional photochemical reactions has relied on imposing geometric constraints on reactants or transition states via conducting photochemistry in organized or constraining media. In our group, we study a fundamentally different approach to affect the course of photochemical reactions by utilizing spatially selective excitation of specific electronic transitions with plane-polarized light in reactant molecules uniformly aligned in the nematic liquid crystal phase. This method represents a new paradigm in controlling photochemical reactivity and increasing the selectivity of photochemical reactions. It also offers a powerful tool to study mechanisms of photochemical transformations.

Presidential Research, Scholarship and Artistry Professor
La Tourette Hall 318
815‐753-6844
een@niu.edu
Educational Background
B.S. – Moscow State University (1992)
Ph.D. – Moscow State University (1996)
Postdoc – Univ. of Wisconsin Madison (1998-2002), Massachusetts Inst. of Technology (2002-2004)
Research Interests
Experimental and theoretical organic chemistry, functional organic materials and polymers, physical organic chemistry and photochemistry.