Timothy J. Hagen
Design and Synthesis of Molecules with Biological Significance
Research interests in our laboratory are at the interface of chemistry and biology. The focus is on structure‐based design and synthesis of small molecules that can modulate essential pathways of infectious disease organisms with an emphasis on small molecule–protein interactions and target specificity. Our laboratory utilizes fragment‐based screening, de novo design, the design and synthesis of versatile fragment building blocks from natural products and construction of fragment libraries to explore enzyme selectivity.
Specific areas of interest include: (1) Fragment‐based design of novel small‐molecules to selectively disrupt key enzymatic interactions in the non‐mevalonate isoprenoid biosynthetic pathway; (2) Design of novel small‐molecules to selectively inhibit the P. falciparum and bacterial forms of enzymes such as MetAP2; (3) Natural product synthesis with investigation of their biological activities. Our group uses a multidisciplinary approach toward achieving these research goals including synthetic organic chemistry, computer modeling, protein crystallography, molecular and cell biology.
Fragment based drug discovery is a rapidly growing technique in medicinal chemistry, that utilizes protein crystallography to discover unique fragments that bind to protein targets of biological interest. These low molecular weight fragments can then be optimized using medicinal chemistry techniques to obtain new compounds with low nM potencies. This can be achieved with a limited number of compounds, especially if good structural data is present. These techniques are being applied to enzymes in the non‐mevalonate isoprenoid biosynthetic pathway. We have established collaborations with research groups that have excellent structural capabilities and experience.
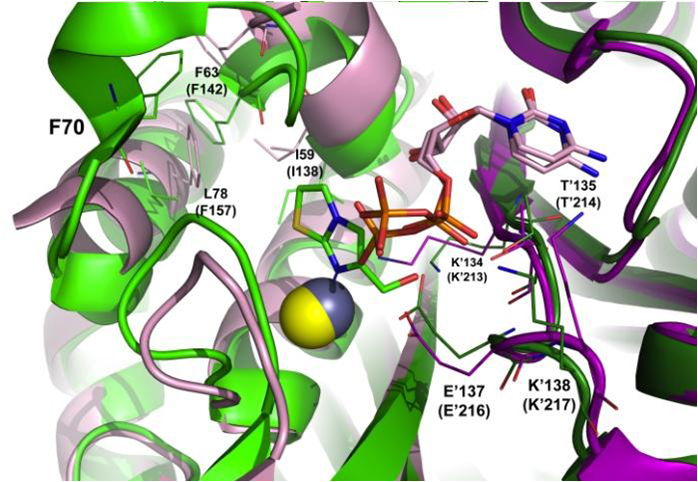
Figure 1. Trimeric crystal structure of BpIspF bound to FOL717 (light/dark green; PDB code 3IKF) with zinc atom (gray) aligned with two monomeric copies PfIspF bound to CDP (pink/magenta; PDB code 4C81) with catalytic zinc atom (yellow).
Certain enzymes are essential to bacterial and parasitic organisms such a R. prowazekii and P. falciparum and our approach is to use synthetic organic chemistry, medicinal chemistry and structural biology to design small molecule inhibitors of these enzymes. Methionine Aminopeptidase (MetAP) is a metalloprotease enzyme that removes the N‐terminal methionine from nascent proteins and it is essential to humans and infectious disease causing organisms. The human forms of MetAP1 and MetAP2 enzymes have been well studied however, the MetAP1 form of R. prowezkii and P. falciparum form of MetAP2 have not. We are designing and synthesizing new molecules that will have potency and selectivity for the R. prowazekii MetAP1 and P. falciparum MetAP2. These compounds will be useful for treating typhus, malaria and other infectious disease.
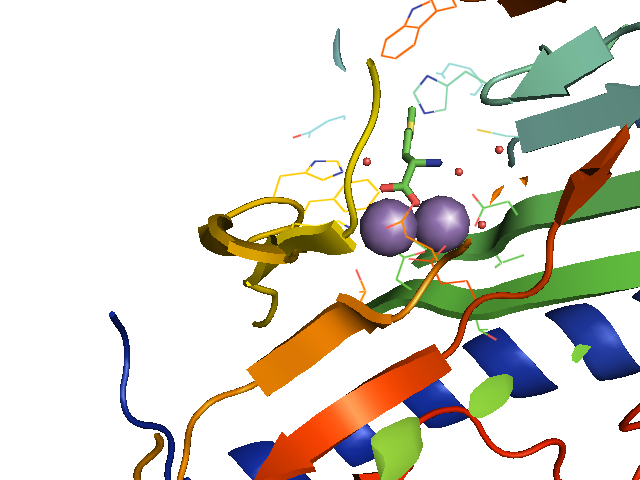
Figure 2. R. prowazekii methionine aminopeptidase 1 in complex with methionine (green). (PDB 3MX6)
We are interested in synthetic methodology to natural products, such as the bengamides, that demonstrate interesting biological activity. The bengamides show anticancer, antibiotic and antihelmintic properties. pergent synthetic routes to bengamide analogs will allow for rapid synthesis of novel analogs that may lead to improved treatments for infectious disease including malaria.
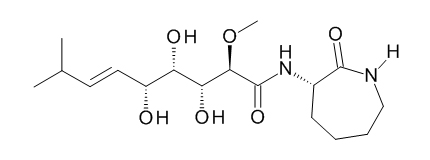
Figure 3. Structure of bengamide E.
Representative publications
- Esan, T.E., Lail, C.L., Daraji, D.G., Krysan, D.J., and Hagen, T.J. (2024). "An improved synthesis of alkyl AMP esters." Tetrahedron Letters, 151, 155329. https://doi.org/10.1016/j.tetlet.2024.155329
- Jezewski, A.J., Esan, T.E., Propp, J., Fuller, A.J., Daraji, D.G., Lail, C.L. III, Staker, B.L., Woodward, E.L., Liu, L., Battaile, K.P., Lovell, S., Hagen, T.J., and Krysan, D.J. (2024). "A single Leishmania adenylate-forming enzyme of the ANL superfamily generates both acetyl- and acetoacetyl-CoA." The Journal of Biological Chemistry, 107879. https://doi.org/10.1016/j.jbc.2024.107879
- Pierce, P.G., Hartnett, B.E., Laughlin, T.M., Blain, J.M., Mayclin, S.J., Bolejack, M.J., Myers, J.B., Higgins, T.W., Dranow, D.M., Sullivan, A., Lorimer, D.D., Edwards, T.E., Hagen, T.J., Horn, J.R., and Myler, P.J. (2024). "Crystal structure and biophysical characterization of IspD from Burkholderia thailandensis and Mycobacterium paratuberculosis." Acta Crystallographica Section F: Structural Biology Communications, 80(2), 43–51. https://doi.org/10.1107/s2053230x24000621
- Sharma, I., Daraji, D., Horn, J.R., and Hagen, T.J. (2024). "Inhibitors of Rickettsia prowazekii methionine aminopeptidase 1 identified from the Pandemic Response Box." Bioorganic & Medicinal Chemistry Letters, 112, 129931. https://doi.org/10.1016/j.bmcl.2024.129931
- Sharma, I., Chen, C., Daraji, D., Horn, J.R., and Hagen, T.J. (2023). "Novel inhibitors of Rickettsia prowazekii methionine aminopeptidase from the Malaria Box." Bioorganic & Medicinal Chemistry Letters, 87, 129281. https://doi.org/10.1016/j.bmcl.2023.129281
- DeBouver, N.D., Bolejack, M.J., Esan, T.E., Krysan, D.J., Hagen, T.J., and Abendroth, J. (2023). "Bacterial structural genomics target enabled by a recently discovered potent fungal acetyl-CoA synthetase inhibitor." Acta Crystallographica Section F: Structural Biology Communications, 79(6), 137–143. https://doi.org/10.1107/s2053230x23003801
- Ndegwa, F.K., Kondam, C., Aboagye, S.Y., Esan, T.E., Waxali, Z.S., Miller, M.E., Gikonyo, N.K., Mbugua, P.K., Okemo, P.O., Williams, D.L., and Hagen, T.J. (2022). "Traditional Kenyan herbal medicine: Exploring natural products’ therapeutics against schistosomiasis." Journal of Helminthology, 96, e16. https://doi.org/10.1017/s0022149x22000074
- Blain, J.M., Grote, D.L., Watkins, S.M., Goshu, G.M., Muller, C., Gorman, J.L., Ranieri, G., Walter, R.L., Hofstetter, H., Horn, J.R., and Hagen, T.J. (2021). "Structural and biophysical characterization of the Burkholderia pseudomallei IspF inhibitor L-tryptophan hydroxamate." Bioorganic & Medicinal Chemistry Letters, 48, 128273. https://doi.org/10.1016/j.bmcl.2021.128273
- Jezewski, A.J., Alden, K.M., Esan, T.E., DeBouver, N.D., Abendroth, J., Bullen, J.C., Calhoun, B.M., Potts, K.T., Murante, D.M., Hagen, T.J., Fox, D., and Krysan, D.J. (2021). "Structural characterization of the reaction and substrate specificity mechanisms of pathogenic fungal acetyl-CoA synthetases." ACS Chemical Biology, 16(8), 1587–1599. https://doi.org/10.1021/acschembio.1c00484

Professor
Faraday Hall 350
815‐753‐1463
thagen@niu.edu
niu.edu/hagen
Educational Background
Postdoctoral Research Fellow, CNS and Drug Design, Searle, 1988–1989
Ph.D., University of Wisconsin—Milwaukee, 1988
B.S., Illinois State University, 1982
Research Interests
Organic Synthesis; natural product synthesis; medicinal chemistry; small molecule protein interactions; drug design.